Abstract

Background: In the POLARIX study (NCT03274492), Pola-R-CHP demonstrated significantly improved progression-free survival (PFS) vs previous standard of care rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP; stratified hazard ratio [HR], 0.73; 95% confidence interval [CI]: 0.57-0.95; p=0.02), with a similar safety profile in patients (pts) with previously untreated DLBCL (Tilly H, et al. N Engl J Med 2022). Here we report an ad hoc analysis to evaluate the potential impact of Pola-R-CHP on 2L treatment, using results of the POLARIX study projected to the real-world DLBCL population.
Methods: Data from pts across six countries were derived from POLARIX (data cut-off: June 28, 2021). 2L treatment was defined as the first treatment after progression, or treatment change due to toxicity or other reasons, whichever occurred first. 2L treatments were grouped into six categories: autologous stem cell transplant (ASCT), chemotherapy only (chemo), rituximab plus chemotherapy (R-chemo), radiotherapy (RT), clinical trials, and other therapy. Cumulative incidence of 2L treatment as a function of time was estimated using Fine-Grey methodology, with death as a competing risk; long-term extrapolation outcome was estimated using the mixture cure rate models fitted on PFS and overall survival data. A dynamic multi-cohort partition survival model was constructed to estimate population-level impact of first line (1L) DLBCL treatment with Pola-R-CHP on the incidence of 2L treatment, over a 10-year period (2023-2033). DLBCL population incidence (2018-2032) was based on age-specific rates taken from country-specific registries and applied to population counts for each age group using United Nations population data (United Nations. World Population Prospects 2019). The population distribution of International Prognostic Index (IPI) score 2-5 was assessed from literature and reflected 70% of diagnosed pts (Harrysson S, et al. Blood Cancer J 2021; Tuominen S, et al. Future Oncol 2022). DLBCL population incidence counts for IPI 2-5 were imputed into the dynamic model to parse the effects between treatment with Pola-R-CHP and R-CHOP.
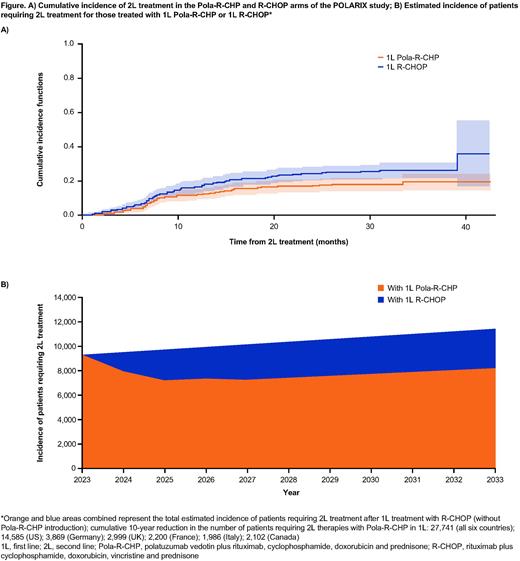
Results: Among pts who received 2L treatment in POLARIX (Pola-R-CHP, n=78; R-CHOP, n=109), the majority (84%) in both arms required subsequent treatment due to disease progression. In line with the previously reported PFS benefit, pts in the Pola-R-CHP arm received fewer 2L treatments than pts in the R-CHOP arm, with a 34% risk reduction (subdistribution HR, 0.66; 95% CI: 0.49-0.88); cumulative incidence of 2L treatment at 24 months was 17.1% (95% CI: 13.5-20.6) vs 24.4% (95% CI: 20.4-28.5), respectively (Figure A). At baseline, risk factors for requiring 2L treatment, after adjustment for treatment effect, were: Ann Arbor Stage III-IV, bulky disease, and double expressor lymphoma (DEL). Mortality as a competing risk to 2L was similar across both treatment arms. As expected, the types of 2L therapies used were similar between the Pola-R-CHP and R-CHOP arms of POLARIX (Pola-R-CHP vs R-CHOP, n [%]: ASCT, 16 [20.5] vs 25 [22.9]; R-chemo, 39 [50.0] vs 53 [48.6]; chemo, 5 [6.4] vs 7 [6.4]; RT, 10 [12.8] vs 12 [11.0]; clinical trials, 3 [3.8] vs 8 [7.3] [including chimeric antigen receptor T-cell therapy, n=2 vs n=1, respectively], and other therapy, 5 [6.4] vs 4 [3.7], respectively), suggesting that the use of Pola-R-CHP instead of R-CHOP in 1L DLBCL does not impact choice of 2L treatment strategy. In the real-world setting, replacing R-CHOP with Pola-R-CHP as 1L treatment in pts with IPI 2-5 would translate to a 27% cumulative reduction of 2L treatments during the 10-year period analyzed (Figure B). For the six countries studied, this would result in a total of 27,741 additional pts avoiding the burden of later-line therapies.
Conclusions: Results demonstrate that 2L therapies used were similar between pts who received Pola-R-CHP vs R-CHOP, suggesting 1L use of Pola-R-CHP does not alter subsequent therapy selection. Moreover, analysis of the potential impact on 2L treatment in the next 10 years showed that Pola-R-CHP reduced the risk of undergoing 2L treatment compared with R-CHOP by 27%, including pts with high-risk tumor subsets such as DEL, translating to 27,741 pts across the countries studied. Further study with longer follow up is needed to determine the number of treatments that would have been avoided by these pts in the third-line setting and beyond.
Disclosures
Boissard:Hoffmann La Roche: Current Employment. Tilly:ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees. Lenz:Miltenyi: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria; Constellation: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Genmab: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria, Research Funding, Speakers Bureau; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Bayer: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; University Hospital Münster: Current Employment. Collins:BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Speakers Bureau; SecuraBio: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants / expenses, Speakers Bureau; Gilead: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants / expenses, Speakers Bureau. Pinto:Merck Sharp and Dohme: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier Affaires Medicales: Honoraria; F. Hoffmann-La Roche AG, Incyte (Italy), Merck Sharp and Dohme, Servier Affaires Medicales: Honoraria; F. Hoffmann-La Roche AG, Merck Sharp and Dohme, Incyte (Italy): Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche AG: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees. Sehn:Teva, Roche/Genentech: Consultancy, Honoraria, Research Funding; AbbVie, Acerta, Amgen, Apobiologix, AstraZeneca, BMS/Celgene, Debiopharm, Genmab, Gilead, Incyte, Janssen, Kite, Karyopharm, Lundbeck, Merck, Morphosys, Novartis, Sandoz, Seattle Genetics, Servier, Takeda, TG Therapeutics, Verastem: Consultancy; Chugai: Consultancy, Honoraria; AbbVie, Acerta, Amgen, Apobiologix, AstraZeneca, BMS/Celgene, Gilead, Incyte, Janssen, Kite, Karyopharm, Lundbeck, Merck, Morphosys, Sandoz, Seattle Genetics, Servier, Takeda, TG Therapeutics, Verastem: Honoraria. Salles:Roche/Genentech, Gilead Sciences, Janssen, Celgene, Novartis, MorphoSys AG, Epizyme, Alimera Sciences, Genmab, Debiopharm Group, Velosbio, Bristol-Myers Squibb, BeiGene, Incyte, Miltenyi Biotec, Ipsen, Kite, a Gilead Company, Loxo, Rapt: Consultancy; AbbVie, BeiGene, Bristol Myers Squibb, Celgene, Debiopharm, Epizyme, Genentech/Roche, Genmab, Incyte, Kite, a Gilead Company, Miltenyi, MorphoSys, Takeda, and VelosBio: Membership on an entity's Board of Directors or advisory committees; Roche/Genentech, Janssen, Celgene, Gilead Sciences, Novartis, AbbVie, MorphoSys AG, Amgen, Bayer, Epizyme, Regeneron, Kite, a Gilead Company: Honoraria. Roussel:F. Hoffmann-La Roche: Current Employment, Current equity holder in publicly-traded company. Simonella:Hoffmann-La Roche: Current Employment. Ho:F. Hoffmann-La Roche Ltd: Current Employment. Hirata:Roche: Current holder of stock options in a privately-held company; Genentech, Inc.: Current Employment. Lee:Roche: Current equity holder in publicly-traded company; Genentech: Current Employment. Masaquel:Genentech, Inc.: Current Employment; Roche: Current equity holder in private company, Current holder of stock options in a privately-held company. Launonen:Hoffmann la Roche: Current Employment, Ended employment in the past 24 months.
OffLabel Disclosure:
Polatuzumab vedotin (Polivy) is a CD79b-directed antibody-drug conjugate indicated in combination with bendamustine and a rituximab product for the treatment of adult pts with relapsed or refractory DLBCL, not otherwise specified, after at least two prior therapies.Rituximab (Rituxan) is a CD20-directed cytolytic antibody indicated for the treatment of adult pts with: relapsed or refractory, low grade or follicular, CD20-positive, B-cell NHL as a single agent; previously untreated follicular, CD20-positive, B-cell NHL in combination with first-line chemotherapy (chemo) and, in pts achieving a CR or PR to a rituximab product in combination with chemo, as single-agent maintenance therapy; non-progressing (including stable disease), low-grade, CD20-positive, B-cell NHL as a single agent after first-line CVP chemo; previously untreated diffuse large B-cell, CD20-positive, NHL in combination with CHOP or other anthracycline-based chemo regimens; previously untreated and previously treated CD20-positive CLL in combination with fludarabine and cyclophosphamide.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal